
Consortium ALT-SPF
Jürgen Vollhardt, Global Head of Science & Promotion Sun Care at DSM, Member of ISO TC217/WG7 for SNV / and Uli Osterwalder, Principal and Owner at Sun Protection Facilitator GmbH, Chair of ISO TC217
DSM / Sun Protection Facilitator GmbH
Recently, a call for expression of interest to join the ALT-SPF consortium was launched. Can you explain in short words what this is all about?
J. Vollhardt: The ALT-SPF consortium aims to gather a critical mass of suncare industry companies and alternative test developers around the world. With this initiative, we want to combine the resources of our industry to realize a long-cherished wish to characterize new alternative SPF methods sufficiently in terms of accuracy (trueness and precision) in comparison to the in vivoSPF as defined by the ISO 24444 gold standard. Alternative measurements should neither over- nor underestimate the SPF values. This will allow bodies such as ISO to validate such methods and recommend them for general or specific use. We also want to give all relevant alternative methods a chance to compare themselves with the Gold Standard. The call of expression of interest is the first step in bringing all important actors together. Other important actors, such as in vivotest institutes, will be involved in a second step.
In your opinion, what are the weaknesses of ISO standard 24444:2019?
U. Osterwalder: First of all, the SPF in vivo test measures a very important number that characterizes sunscreen performance on human skin. The SPF test is close to the actual use of the products. The measured protection against sunburn (erythema) is also meaningful in terms of protection against skin cancer. Therefore, new methods should be as close as possible to the values of this gold standard. However, today's test is lengthy and it can take up to several weeks to get the result, depending on the amount of test results at the testing institute. This hardly helps a sunscreen manufacturer during development. So, the goal of the ALT-SPF consortium is also to have something faster. In addition, the radiation dose delivered by an alternative method should be negligible.
Who would you like to reach with the ALT-SPF project?
J. Vollhardt: The ALT-SPF consortium is aimed at the entire sunscreen industry and those who are involved in it. First and foremost, the users of the SPF claim, i.e. the sunscreen manufacturers who have an interest in how the SPF will be measured in the future. But it is also an interesting topic for suppliers of UV filters and ingredients in sun protection, such as DSM. We will of course also involve test institutes with conventional or alternative methods, as well as Sun Care experts who can and want to contribute. We do not want to miss an interesting method with potential. And for the alternative methods it is a unique chance to demonstrate their performance and find global recognition.
Isn't there already data showing a match?
U. Osterwalder: Of course, we assume that there is and hope that the method developers will be able to show such data. A lot has been published about the different methods, for example about in vitrotransmission, in vivo/in vitroHybrid Diffuse Reflectance (HDRS) or in silico simulation. But it turns out that the devil is always in the details. There are various sources of error, which sometimes cancel each other out randomly. But this coincidence can also work against a method. But consumer safety and the success of a method should not be based on chance. Therefore, a test procedure is needed that clearly checks the weaknesses of a method and does not just test degrees of error in a generalized way, as the usual correlation diagrams do. For validation purposes it needs to be known where the differences between gold standard and alternative method actually originate from. There are nowadays statistical procedures available that can separate the different sources of bias from each other and quantify them.
What impact do you expect from the results of the ALT-SPF project?
U. Osterwalder: The characterization of alternative methods relative to the gold standard will deliver data to enable a validation process defining the scope for which the alternative methods would work. For this criteria need to be agreed on. A replacement of the good old in vivo method could make development faster as it allows for in between checkpoints. Market surveillance by authorities could also utilize the new methods more broadly.
How can interested partners contribute to the project?
J. Vollhardt: There will be a lot of knowledge necessary, e.g. about different types of samples, specific needs of the industry and also legal aspects of the consortium that require expertise. Currently, experienced method validation statisticians are already on board and suggested an experimental design together with the analysis of the data. That needs to be further worked on. The sophisticated experimental design in the end will be delivering detailed features of the alternative methods, but it still needs to be very efficient. Nevertheless, we expect significant testing efforts. To finance the whole project, we need more sponsors. We are thinking especially of sunscreen manufacturers. The test institutes will make their contribution in the form of measurements. Of course, we also welcome other important stakeholders that want bring forward this activity. Many experts are already involved in ISO TC217 WG7 (Sun Protection). The ALT-SPF consortium is planning a formal liaison with ISO in order to work even closer together, as e.g. Cosmetics Europe has been doing for a long time (see Figure).
Where can you find more information about the project?
J. Vollhardt: The central source of information is currently our "Consortium ALT-SPF" webpage: www.alt-spf.com where the call for expression of interest to join the consortium is open until 1 September. There you will find a letter for participation and a form to fill in in which way you want to support the consortium e.g. as a sponsor and/or through in-kind contribution.
What is the role of the ALT-SPF Consortium with regards to ISO?
U. Osterwalder: ISO develops international standards on a voluntary basis. The experts come mainly from industry, but also from other stakeholders such as authorities or consumer organizations. The effort is paid for by everyone. A well-founded validation, which is in everyone's interest, means an extra effort. This is where the ALT-SPF consortium comes in. The Consortium ALT-SPF wants to ensure that the characterization is carried out according to scientific standards and that every relevant alternative method gets a chance. The resulting data will be configured to allow for validation by interested authorities or standardization bodies. The figure illustrates this role.
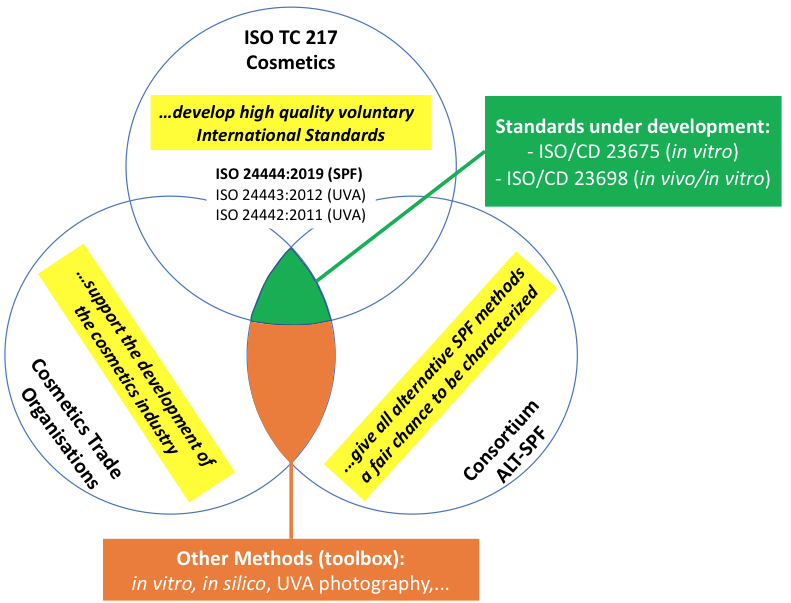
Figure: Role of Consortium ALT-SPF and cooperation with ISO TC 217 and Cosmetics Europe
What is the timetable?
J. Vollhardt: The ALT-SPF consortium is now in the formal setup phase. Once the Consortium Agreement is signed, the samples will be distributed to the testing institutes and the actual testing phase can begin. This will happen in the last quarter of 2020. The tests are scheduled to last six months. This means that in the second quarter of 2021 the results can be summarized in a report. They will then be published in a scientific journal, i.e. in about a year's time, if everything goes according to plan.
Why should a personal care company become a sponsor the ALT-SPF consortium?
J. Vollhardt and U. Osterwalder: As a personal care company sponsoring this initiative, you:
- get access to a huge amount of data on the characterization of alternative SPF test methods
- will get to know the performance of the new methods in detail and for different product categories firsthand and can make your technology selection for your claiming needs
- can interact with ISO experts working on the alternative methods
- can spell out your expectation regarding bias and precision
- will understand why the characterization method would withstand court challenges as it will be done according to the latest technology in statistics and various norms published or in preparation.
- will help providing the industry with a fast and reliable method that will also support your development process better
- will support the in-depth investigation of the methods down to sample specific bias, their variation features relative to in vivoand that way defining properly the applicability and the scope of the new methods.
. . .
This interview is the personal opinion of the interviewed persons and not an official statement of the ISO committee.




